CIMZIA
Price range: $1,499.00 through $1,999.00
CIMZIA® (Certolizumab Pegol) is a targeted anti-TNF biologic therapy designed to treat chronic inflammatory conditions like rheumatoid arthritis and Crohn’s disease. Its innovative formulation provides long-lasting relief while ensuring effective symptom management.
Brand: CIMZIA®
Manufacturer: UCB Canada Inc.
Active Substance: Certolizumab Pegol
Strength: 200 mg/ml
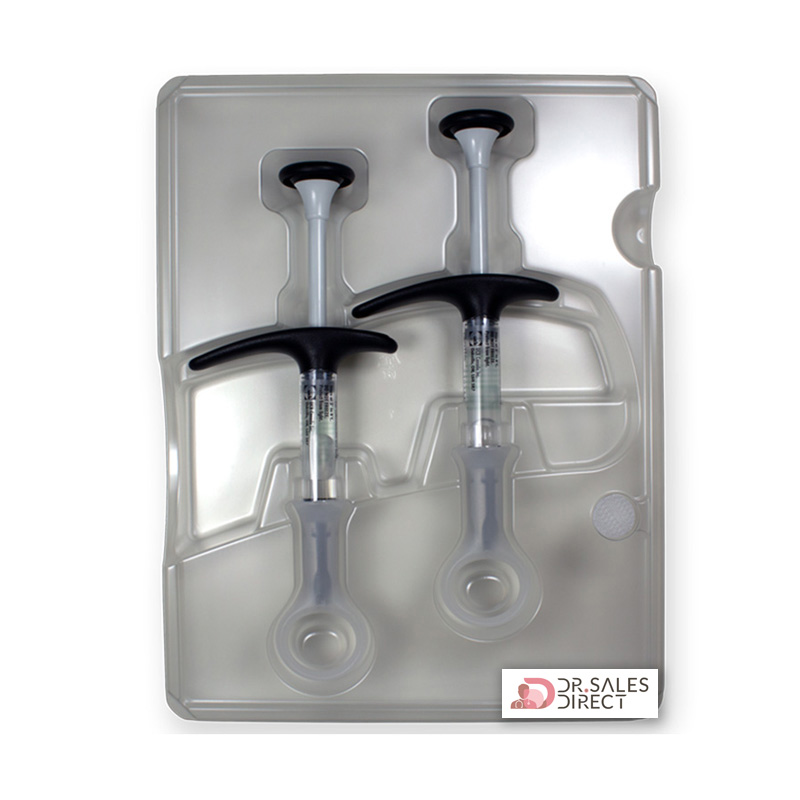
Pack Size: 2 single-use pre-filled syringes (1 ml each) + 2 alcohol swabs
Additional information
| Language | English, Non-English |
|---|
Cimzia is a brand-name prescription medication. It’s used to reduce inflammation (swelling) associated with several long-term conditions. Cimzia is FDA-approved in adults for these conditions:
- Plaque psoriasis (the most common form of the skin condition psoriasis). Cimzia is used to treat moderate to severe plaque psoriasis that could benefit from systemic treatment (treatment that affects the whole body) or phototherapy (light treatment that treats or affects just the skin).
- Crohn’s disease (a type of inflammatory bowel disease). Cimzia is used to lessen signs and symptoms of moderate to severe Crohn’s disease that’s active (causing symptoms). It’s used when other treatments haven’t helped enough.
- Rheumatoid arthritis (a disease that causes joint swelling and damage). Cimzia is used to treat moderate to severe rheumatoid arthritis that’s active.
- Psoriatic arthritis (a type of joint swelling that can sometimes happen in people with psoriasis). Cimzia is used to treat psoriatic arthritis that’s active.
- Ankylosing spondylitis (a kind of joint swelling that affects the spine). Cimzia is used to treat ankylosing spondylitis that’s active.
- Non-radiographic axial spondyloarthritis (a type of joint swelling in the spine or pelvis that causes pain and stiffness but that can’t be seen on an X-ray).
Cimzia is used to treat active non-radiographic axial spondyloarthritis in people who have signs of inflammation in blood tests or MRI scans (pictures of the inside of your body using magnets and radio waves).
Cimzia is a biologic drug (a medication made using living cells). It’s a type of drug called a tumor necrosis factor (TNF) blocker.
Cimzia is given by subcutaneous injection (under the skin). The injection comes in two forms: as a single-dose vial and a single-dose prefilled syringe.
Safety Information
CIMZIA (Certolizumab Pegol) is administered subcutaneously under the supervision of a qualified healthcare provider. Patients with known hypersensitivity to certolizumab pegol or any excipients should not use CIMZIA®. Common side effects may include injection site reactions, upper respiratory infections, headaches, and nausea. Serious but rare adverse events, such as severe infections, tuberculosis reactivation, or hypersensitivity reactions, may occur. Patients should be screened for latent tuberculosis before starting treatment and monitored for signs of infection throughout therapy. CIMZIA® is not recommended for pregnant or breastfeeding women unless clearly necessary. For proper storage, refrigerate the pre-filled syringes at 2°C–8°C and protect them from light.
Pricing Information
To access specific pricing details and complete your purchase, sign in to view detailed wholesale rates.